Specificity : 296/305×100%=97.0%
Brand:
DEEPBLUEIntended use
Test principle
Product advances
1.Pre-filled buffer solution, easier operation.
2.Passed the PEI evaluation.
3.Room temperature storage.
4.No need instrument, get results within 15 minutes.
5.Identify acute or early infection.
6.No reduction in sensitivity test against the Alpha, Beta, Delta, Gamma, Lambda, Omicron variant and so on.
Materials and Components
1.Instruction
2.Packaged test cassette
3.Disposable sampler
4.Buffer
5.Sterile Lancet
6.Alcohol Swab
7.Sterile Pad
8.Collection Bag
9.(Materials required but not provided)
Product Information
| Specification | 1pcs/box,5pcs/box,25pcs/box |
| Specimen | Serum,plasma,whole blood or fingertip blood |
| Storage | 4-30℃ |
Test Procedure
Allow test device extraction reagent and specimens to equilibrate to room temperature(15 ~ 30 ℃) prior to testing.Please keep the temperature at 15 ~ 30 ℃ and the humidity at 20%-80% during the whole test.
1.Specimen Collection
(1)Wipe the fingertips with an Alcohol Swab
(2)Pull out the cap on the front end ofthe lancet. Use sterile lancet to gently press on the wiped area
(3)Squeeze out a sufficient amount of blood
(4)Pinch the bulb at the upper end of the disposable sampler, place the lower end vertically in the blood, and then slowly loosen the bulb. The blood must exceed the thin tube, asshown in the figure as an example.
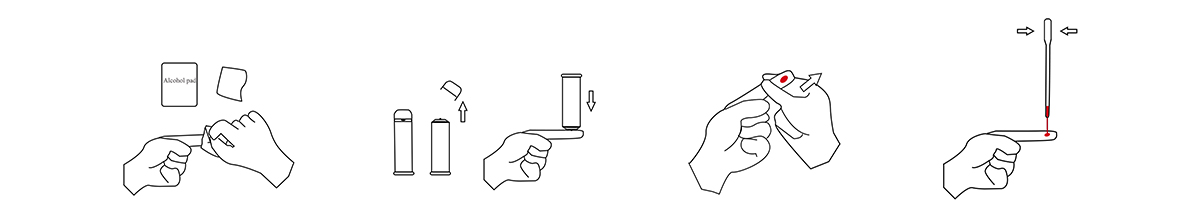
2.Testing
(1)Add a drop of blood to the sample well(S).
(2)Break off the bottleneck of thebuffer solution and squeeze adrop of buffer solution into the sample hole. Start the timer.

3.Test Results
Read result at 15 minutes.Do not read results after 30minutes.
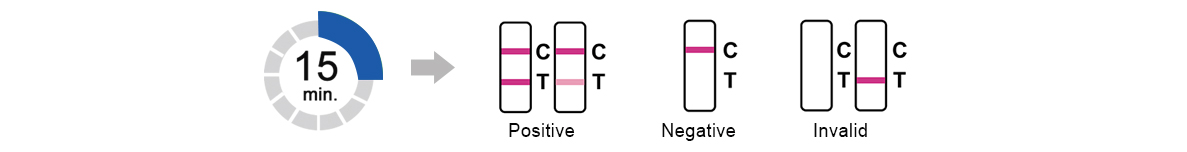
Clinical Performance
| Assessment reagent |
Comparison method
|
Total (example)
|
|
|
Positive (case)
|
Negative (case)
|
||
|
Positive
|
279 | 21 | 300 |
|
Negative
|
9 | 296 | 305 |
|
Total
|
288 | 317 | 605 |
Sensitivity : 279/300×100%=93.0%
Specificity : 296/305×100%=97.0%